Solved Question Papers for Science Term 2 Set 1
SECTION – A
Question 1.
The table shows the electronic structures of four elements.
| Element | Electronic Structure |
| P | 2,6 |
| Q | 2, 8,1 |
| R | 2, 8,7 |
| S | 2, 8,8 |
(A) Identify which elements) will form covalent bonds with carbon.
Answer:
P and R
Detailed Answer:
The number of electrons are either lost or gained or shared by one atom of an element to achieve the nearest inert gas electronic configuration, gives us the valency of the eLement.
From the table, it is clear that P and R are non-metals. We know covalent bonds are formed between non-metals by sharing the electrons. In case of ‘P’ we see the formation of a double bond between two P atoms. An atom of ‘P’ has 6 electrons in L shell and it requires two more electrons to complete the octet as that of the nearest gas neon so each atom of ‘P’ shares two electrons with another atom of’P’ to give the structure?
The two electrons contributed by each ‘P’ atom give rise to two shared pairs of electrons so a double bond is formed two P atoms and ‘P’ is divalent i.e. P2. P shares two electrons so its valency is 2.
In case of ‘R’, it has 7 electrons in its M shell and it requires one more electron to complete its octet as that of the nearest gas argon. So two ‘R’ atoms share their electrons to form a molecule of ‘R’ i.e. R2. The electron dot structure of’R’ molecule is:
The shared pair constitutes a single covalent bond between two ‘R1 atoms and is represented by a single line (-). ‘R’ shares only one electron so its valency is 1.
In case of element ‘Q’ it has one valence electron, it is a metal, it can attain its stable configuration by losing one electron and form ionic or electrovalent bond Q+ + e–.
‘S’ has already its stable configuration; it cannot gain or lose or share the electron and its valency is zero so it is an inert gas.
Related Theory
The two pairs of electrons on each P atom and three paurs of electrons on each R atom are not involved in bond formation and are called unshared pairs or lone pairs. The elements P and R are oxygen and chlorine atoms.
(B) “Carbon reacts with an element in the above table to form several compounds.” Give suitable reason.
Answer:
Carbon has a valency four or Tetravalency & Catenation
Carbon is a non-metal with atomic number (z) equal to 6. Its electronic configuration is 2, 4. Thus, carbon has four valence electrons. It does not take part in the ionic bond formation as it can neither gain nor lose four electrons. Therefore, carbon completes its octet by sharing four electrons present in the valence shell with the electrons of other atoms. This means that carbon is tetravalent or has a valency of four. Carbon has the unique ability to form bonds with other atoms of carbon, gives rise to large molecules and the property is called catenation, Carbon can react with P and forms CP2.
Carbon can also react with R and forms CR4
Question 2.
The diagram below shows part of the periodic table.
(A) Which elements would react together to form covalent compounds?
(b) Between the two elements W and Z, which will have a bigger atomic radius? Why?
Answer:
(A) Y and Z
(B) W is bigger,
Reason:
Down the group number of shells increases
Detailed Answer: (A) In order to understand the reactivity of the given elements, we need to first find out the group and period of all elements.
As we can see from the above table ‘W’ belongs to second group and is a metal (metals are on the Left side of the modern periodic table). T and ‘Z’ are non-metals and belong to group 16 and 17 respectively. We know only non-meals react to form covalent compounds. Hence, elements T and ‘Z’ would react together to form covalent compounds.
‘Y’ belongs to group 16 and period 3 where ‘Z’ belongs to group 17 and period 2.
‘Y’ has 6 valence electrons and ‘Z’ has 7 valence electrons. Each ‘Z’ atom needs one electron to complete its octet and Y atom needs two electrons to attain stable configuration so each Y atom shares its two electrons with two ‘Z’ atoms to form Z2Y.
The electron dot structure is given below:
Related Theory
‘W’ is metal and it can form electrovalent bond with Z and Y. Their formulae will be WZ and WY2.
(B) Element W would have a bigger atomic radius than ‘Z’. Element ‘Z’ belongs to period 2 and ‘W’ belongs to period 4. The number of shells are more in W. Moreover, the size decreases as we move from left to right in a period due to effective nuclear charge. Increase in nuclear charge tends to pull the electrons closer to the nucleus and reduces the size of the atoms. Hence ‘Z’ is smaller in size.
The atomic size increases down the group as new shells are being added as we go down the group. This increases the distance between the outer most electrons and the nucleus. Thus ‘W’ has a bigger atomic radius than ‘Z’ as well as Y.
Related Theory
The atomic size refers to the radius of an atom. The atomic size is the distance between the centre of the nucleus and the outermost shell of an isolated atom and its unit is pm.
Question 3.
(A) Trace the path a male gamete takes to fertilise a female gamete after being released from the penis.
Answer:
Male gamete (sperm) travels in the female reproductive tract after being released. The path which it takes to fertilise the female gamete (egg) is vagina uterus, fallopian tube resulting in a zygote;
Alternatively accept the labelled figure of human female reproductive system indicating the passage of sperm from vagina to uterus bhd then to fallopian tube for fertilisation resulting in a zygote;
Detailed Answer:
A male gamete (sperm) produced in the testes of human males are introduced into the vagina of the female through penis during sexual intercourse. In this way, millions of male gametes are released into the vagina at a time. The sperms are very active and motile due to the presence of tail. The sperms move through vagina, the cervix into the uterus.
From uterus the sperms pass into the fallopian tube
Penis → Sperm → Vagina → Cervix → Uterus → Fattopian tube.
One of the (oviducts) fallopian tubes contains an ovum released by the ovary during ovulation. Only one sperm fuses with the ovum in the fallopian tube to form a zygote.
(B) State the number of sets of chromosomes present in a zygote.
Answer:
Zygote has 2 sets of chromosomes alternatively accept 2n. No marks to be assigned for n or 3n.
Detailed Answer:
Zygote is a single cell which is formed by the fusion of a male gamete and a female gamete. The zygote has two copies of each chromosome, one each from male and female parents. Every gamete (germ cell) will take one chromosome from each pair and these may be of either male and female parents. When two gametes join, they wilL restore the normal number of chromosomes in the progeny, ensuring the stability of the DNA of the species. Therefore, we can say in a zygote, two sets (2n) of chromosomes are present.
Question 4.
Rajesh observed a patch of greenish black powdery mass on a stale piece of bread
(A) Name the organism responsible for this and its specific mode of asexual reproduction.
Answer:
The greenish black powdery mass on a stale piece of bread is due to bread mould Rhizopus which reproduces by spore formation.
Detailed Answer:
pore formation is an asexual mode of reproduction in bread mould (rhizopus). In spore formation, parent plant produces hundreds of microscopic spores in the sporangia which are reproductive parts. When the sporangium bursts open, the spores spread into the air.
(B) Name its vegetative and reproductive parts.
Answer:
Hyphae or thread like structures are the vegetative part and tiny blob like structures or sporangia are the reproductive parts.
Detailed Answer:
When the spores get a suitable substratum and appropriate conditions (warm and humid atmosphere), the thread like structures (hyphae) are developed which are non-reproductive parts. Thin stems having blob like structures called sporangia which contains spore enclosed in a protective covering are formed. Spores are asexual reproductive units which would reproduce into more bread mould when they get appropriate conditions.
Question 5.
Mustard was growing in two fields- A and B. While Field A produced brown coloured seeds, field B produced yellow coloured seeds.
It was observed that in field A, the offsprings showed only the parental trait for consecutive generations, whereas in field B, majority of the offsprings showed a variation in the progeny.
OR
In an asexually reproducing species, if a trait X exists in 5% of a population and trait Y exists in 70% of the same population, which of the two trait is likely to have arisen earlier? Give reason. (2)
Answer:
In field A, the reason for parental trait in consecutive generations of the offsprings is self-pollination.
In field B, variation is seen to occur because of recombination of genes as cross-pollination is taking place.
OR
Trait Y which exists in 70% (larger fraction) of the population, is likely to have arisen earlier because in asexual reproduction, identical copies of DNA are produced and variations do not occur.
New traits come in the population due to sudden mutation and then are inherited. 70 % of the population with trait Y is likely to have been replicating that trait for a longer period than 5 % of population with trait X.
Detailed Answer:
In field A, the reason for potential trait for consecutive generation of the offsprings is due to self pollination of brown coloured seeds (BB).
Again self pollination of pure line brown seeds were undertaken to produce only brown seeds.
In field B, variations are seen in consecutive generations due to cross pollination between brown coloured seeds and yellow coloured seeds. The genetic material of two combines and the resulting seeds from that cross pollination will have characteristics of both and show new variants.
Case I:
F2 Generation:
| B | b | |
| B | BB Brown |
Bb Brown |
| b | Bb Brown |
bb Yellow |
Variations are seen in second generation.
Phenotypic ratio :
Case II:
| B | b | |
| b | BB Brown |
Bb Brown |
| b | Bb Brown |
bb Yellow |
Phenotypic ratio :
Variants are seen in F1 and F2 generations.
OR
Variations in a species arise either due to errors in DNA copying or during sexual reproduction or due to mutations. But in a sexually reproducing species, no reshuffling of traits occurs. Appearance of a few new traits in the population is due to small inaccuracies during DNA copying. These very few traits wilL be. in very small proportion than the traits already present. Thus, trait Y which exists in 70% of population must have arisen earlier than that of trait X which occurs only in 5% of the population.
Question 6.
A simple motor is made in a school laboratory. A coil of wire is mounted on on axle between the poles of a horseshoe magnet, as illustrated.
In the example above, coil ABCD is horizontal and the battery is connected as shown.
(A) For this position, state the direction of the force on the arm AB.
(B) Why does the current in the arm BC not contribute to the turning force on the coil?
OR
A circuit contains a battery, a variable resistor and a solenoid. The figure below shows the magnetic field pattern produced by the current in the solenoid.
(A) State how the magnetic field pattern indicates regions where the magnetic field is stronger.
Answer:
downwards
OR
Relative closeness of field Lines indicates the strength of magnetic field. Since field Lines are crowded around the ends of the solenoid, hence these are the regions of strongest magnetism.
Detailed Answer:
When current flows through coil, arms AB and CD experience magnetic force. On applying Fleming’s left hand rule, the force acting on arm AB pushes it downwards and arm CD experiences force in the upward direction.
(B) What happens to the magnetic field when the current in the circuit is reversed? (2)
Answer:
Because BC is in the same direction as the direction of field lines. Force is minimum when the direction of current in the conductor is the same as that of the magnetic field. BC wilt not contribute as the force on this part of the coil wilt be cancelled by the force on DA.
OR
The direction of the field will also be reversed.
Related Theory
The reversal in the direction of flow of current through arms AB and CD also reverses the direction of force acting on these arms. The reversing of current in the coil ABCD in repeated after each half rotation and as a result, coil and axle continue to rotate in the same direction. Hence electrical energy is converted to mechanical energy.
OR
Concept Applied :
The pattern of magnetic field lines around a current carrying solenoid is similar to the pattern of the field with the magnetic field around a bar magnet. As it is clear from the figure, the magnetic field lines emerge from north pole and merge at south pole. \Ne know that stronger the magnetic field, closer are the field lines.
Related Theory
As the electric current in each circular loop of solenoid coil flows in the some direction, the magnetic fields of all the loops are added up and as a result, the magnetic field inside the solenoid coil becomes a strong field.
Question 7.
DDT was sprayed in a lake to regulate breeding of mosquitoes. How would it affect the trophic levels in the following food chain associated with a lake? Justify your answer.
OR
In the following food chain, vertical arrows indicate the energy lost to the environment and horizontal arrows indicate energy transferred to the next trophic level. Which one of the three vertical arrows (A, C and E) and which one of the two horizontal arrows (B and D) will represent more energy transfer? Give reason for your answer. (2)
Answer:
(1) DDT being a non- biodegradable pesticide will enter the food chain from the first trophic level i.e Plankton.
(2) Non – biodegradable pesticides accumulate progressively at each trophic level.
This phenomenon is known as biological magnification.
(3) HAWK will have the highest level of pesticide.
OR
A will represent more energy transfer as compared to C and E.
B will represent more energy transfer as compared to D.
When green plants are eaten by primary consumers, a great deal of energy is lost as heat to the environment, some amount goes into digestion and in doing work and the rest goes towards growth and reproduction. An average of 10% of the food eaten is made available for the next level of consumers. This loss of energy takes place at every trophic level.
Alternatively accept – In accordance with 10% law of transfer of energy in a food chain only 10% of energy available at one trophic level is transferred to the next trophic level.
Detailed Answer:
DDT (which is banned in India) is a pesticide which is used in crop fields in an attempt to control pests, These are washed down into the aquatic bodies. From of here, they are absorbed by planktons alongwith minerals and water and in this way, they enter the food chain. Since, they are non-biodegradable, these persist as such for long period of time, when planktons are eaten by small fish. They enter into their bodies and so on they become more and more concentrated at each trophic level. That is why hawk has the maximum concentration of DDT of in this food chain.
Related Theory
In the soil, DDT enters in the food chain at the level of producers. From producers to herbivores and so on. The amount of DDT increases at each successive trophic LeveL. Humans being at the top in the food chain accumulate maximum concentration of toxic non-biodegradable substance in the body.
OR
Related Theory
Green plants in a terrestrial ecosystem capture 1% of energy of sunlight that falls on their leaves and transforms it into food energy by the process of photosynthesis. Deer is heribivore which feeds on green ptants and these herbivores in turn ore eaten up by tiger (carnivore), in this way transfer of energy takes place.
SECTION – B
Question 8.
Choose an element from period 3 of modern periodic table that matches the description given below in each instance. Give reason for your choice.
(A) It has a similar structure to diamond.
Answer:
Silicon
Reason: Tetrahedral structure
OR
Tetravalency or Four valeny and
OR
Covalent bonding like carbon
Detailed Answer: In order to understand and choose an element that matches the description of given elements, first we need to know the position of the elements given and the elements of third period (a, b and c in the boxes are fore questions given)
Silicon (Si) belongs to the same group as diamond and has four valence electrons. Silicon exhibits the property of catenation like carbon (diamond is an allotrope of carbon). Silicon forms compounds with hydrogen which have chains of upto seven or eight atoms but unlike carbon compounds, these compounds are very reactive.
(B) It has same valency as Lithium.
Answer:
Sodium
Reason: It has 1 valence electron like Lithium
Sodium (Na) belongs to the same group I as lithium (Li). Na and Li are alkali metals, have one valence electron and are electropositive in nature.
(C) It has variable valency and is a member of the Oxygen family (group 16).
Answer:
Sulphur
Reason: it forms oxides SO2 and SO2
Sulphur (S) and Oxygen (O) belong to group 16 have 6 valence electrons and in order to achieve stability, sulphur like oxygen needs to gain two electrons. Sulphur lies in period 3 but it can have extended valency which ranges from -1 to +6. In case of SO2 molecule, one atom of sulphur is bonded with two atoms of oxygen. For two atoms of oxygen total four electrons are needed. Therefore in this case (SO2) the valency of ‘S’ is 4. Similarly in SO3, the valency is 6 to satisfy the three oxygen atoms.
Valence shell of S has enough orbitals to accommodate more electrons. It has d-orbitals to accommodate electrons. That is why in SO2, Sulphur shows +4 valency and in SO3, it shown +6 valency.
Related Theory
The atomic size increases down the group because new shells are being added as we go down the group. Sulphur and sodium have more atomic radii than diamond and lithium respectively.
Question 9.
(A) How many isomers are possible for the compound with the molecular formula C4H8? Draw the electron dot structure of branched chain isomer.
(B) How will you prove that C4H8 and C5H10 are homologues?
OR
A carbon compound ‘A’ having melting point 156K and boiling point 351K, with molecular formula C2H6O is soluble in water in all proportions.
(A) Identify ‘A’ and draw its electron dot structure.
(B) Give the molecular formulae of any two homologues of ‘A’.
Answer:
(A) Four
(B) C4H8 and C5H10 are homologues as they differ in:
(1) “- CH2-”
(2) differ in 14u molecular mass
(3) Same functional group
(4) Same general formula
(Any two reasons)
OR
(A) Ethanol; C2H5OH
(B) CH3OH and C3H7OH are homologues of ethanol
OR
CH4O and C3H8O
Detailed Answer:
(A) The organic compounds having the same molecular formula but different structures are known as isomers. C4H8 is butene having general formula CnH2n (Alkene). There are four isomeric alkenes of formula C4H2n.
The isomers of C4H8 are:
(B) A homologous series is a group of organic compounds having similar structures and similar chemical properties in which the successive compounds differ by – CH2 group.. The general fromula of the homologous series of alkenes in CnH2n where n is the number of carbon in one molecule of alkene.
- C4H8 and C5H10 can be represented by the general formula CnH2n.
- C4H8 and C5H10 are two adjacent homologues which differ by 1 carbon atom and 2 hydrogen atoms in their molecular formulae i.e. —CH2.
- The difference in the molecular masses of these two homologues is 14 u.
- These two homologues show similar chemical properties.
- The members of homologous series show a gradual change in their physical properties with increase in molecular mass.
For example as the number of carbon atoms per molecule increases, melting points, boiling points and densities of its members also increase gradually.
OR
(A) A carbon compound ‘A’ having molecular formula C2H6O is ethanol. It is soluble in water in all proportions. C2H6O contains 2 carbon atoms so its parent alkane is ethane C2H6. It also contains alcohol group (—OH group) which is indicated by using -01 so we get the name ethanol:
(B) The preceeding homologue of C2H5OH is CH3OH and succeeding homologue of C2H5OH is C3H7OH. The difference in the molecular formula of CH3OH and C5H5OH is -CH2 and C2H5OH and C3H7OH is also – CH2 which is a characteristic of homologues, molecular mass of CH3OH
= 1 × 12 + 3 × 1 + 1 × 16 + 1 × 1
= 12 + 3 + 16 + 1 = 32u
Molecular mass of C2H5OH
= 2 × 12 + 5 × 1 + 1 × 16 + 1 × 1
= 24 + 5 + 16 + 1 = 46u
Molecular mass of C3H7OH
= 3 × 12 + 7 × 1 + 1 × 16 + 1 × 1
= 36 + 7 + 16 + 1 = 60u
The difference in the molecular mass between the homologues is 14u.
We can call CH3OH, C2H5OH and C3H7OH a homologous series as they differ by -CH2 and their molecular masses differ by 14 u.
Question 10.
Two pea plants – one with round yellow seeds (RRYY) and another with wrinkled green (rryy) seeds produce Fi progeny that have round, yellow (RrYy) seeds.
When F1 plants are self-pollinated, which new combination of characters is expected in F2 progeny? How many seeds with these new combinations of characters will be produced when a total 160 seeds are produced in F2 generation? Explain with reason.
Answer:
- Round green
- Wrinkled yellow
New combinations are produced because of the independent inheritance of seed shape and seed colour traits.
Detailed Answer:
A cross between two plants having two pairs of contrasting characters is called dihybrid cross. One pea plant with round yellow seeds (RRYY) in which RR are the dominant genes for round shape whereas YY are the dominant genes for yellow colour. The other pea plant with wrinkled green seeds (rryy) in which rr are the recessive genes for wrinkled shape whereas yy are the recessive genes for green colour. When two contrasting pairs of traits (RRYY) and (rryy) were crossed, the F1 progeny having (RrYy) round and yellow seeds were formed. In F1 generation only dominant characters were expressed. The other characters (recessive) were not lost even when they are not expressed. When F1 plants were self-pollinated, four types of combinations were seen. These included two parental types and two new combinations.
We can conclude that round and yellow seeds are dominant characters. Occurence of new combinations show that genes for round (seeds shape) and yellow seeds (seed colour) are inherited independently of each other.
Related Theory
Based on hybridisation experiment on garden pea plant, Mendel proposed the lows of inheritance.
- Law of Dominance
- Law of Segregation
- Law of Independent Assortment.
Question 11.
(A) It would cost a man f 3.50 to buy 1.0 kW h of electrical energy from the Main Electricity Board. His generator has a maximum power of 2.0 kW. The generator produces energy at this maximum power for 3 hours. Calculate how much it would cost to buy the same amount of energy from the Main Electricity Board.
Answer:
E = P × T So, E = 3 × 2 = 6 kWh
Cost of buying electricity from the main electricity board = 6 × 3.50 = ₹ 21.0
Detailed Answer:
P = 2.0 kWh
T = 3 hours
Cost of 1 kWh (1 unit) of electrical energy from board = 3.5
E = P × T
= 2.0 × 3 = 6 kWh
Cost of buying electricity from main electricity board = 6 × 3.50 = 21.00
Related Theory
The rate of which electric energy is consumed or dissipated in an electric circuit is power. The commercial unit of electric energy is kWh which is 1 unit of electrical energy.
(B) A student boils water in an electric kettle for 20 minutes. Using the same mains supply he wants to reduce the boiling time of water. To do so should he increase or decrease the length of the heating element? Justify your answer. (3)
Answer:
To reduce the boiling time using the same mains supply, the rate of heat production should be large. We know that P = V2/R. Since V is constant, R should be decreased. Since R is directly proportional to l so length should be decreased.
Detailed Answer:
The new is produced by the heating effect of electric current when electric current flows through the resistant element of the electric kettle the flowing charges suffer resistance.
OR
Related Theory:
Resistance is directly proportional to the length of the conductor, inversely proporitonal to the area of cross section of the conductor. ft is also dependent on the nature of the conductor.
Question 12.
In the above circuit, if the current reading in the ammeter A is 2A, what would be the value of R1?
OR
Calculate the total resistance of the circuit and find the total current in the circuit. (3)
Answer:
5 ohm, 10 ohm and R1 are in parallel
Now, 6 ohm, 6 ohm and Rp are in series
Thus.
Req = 12+10R13R1+10 ………..(1)
V = I Req
From the circuit
Req = 302 = 15 A ……….(2)
Equating (1) and (2)
12+10R1(3R1+10) =15
10R1(3R1+10) = 3
10 R1 = (9 R1 + 30)
Thus, R1 = 30 ohm.
OR
R3 and R4 are in series hence the equivalent resistance of those two = R5 = R3 + R4 = 4 ohms.
R5 and R2 are in in parallel let R6 be the equivalent resistance for them. Hence R6 = R5 + R2 (R5 + R2) = 100/20 = 5 ohms
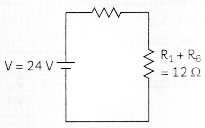
Now R1 and R6 are in series and hence the final equivalent resistance of the entire circuit is R = R1 + R6 = 12 ohms
By Ohm’s law we know that V = IR hence I = V/R. Hence the current in the circuit is 24/12A = 2A
Concept Applied
The formulae used in this question are:
1Rp=1R1+1R2+1R3
Rs = R1 + R2 + R3
Related Theory
When several resistors are joined in parallel the equivalent resistance is less than the value of the smallest individual resistance in the combination. When several resistors are joined in series the resistance of the combination equals the sum of their individual resistances R1 R2 R3 and is thus greater than any individual resistance.
Question 13.
Gas A, found in the upper layers of the atmosphere, is a deadly poison but is essential for all living beings. The amount of this gas started declining sharply in the 1980s.
(A) Identify Gas A. How is it formed at higher Levels of the atmosphere?
Answer:
Gas A is Ozone. Alternatively accept the formula of the gas.
Ozone at the higher levels of the atmosphere is a product of UV radiation acting on oxygen (O2) molecule. The higher energy UV radiations split apart some molecular oxygen (O2) into free oxygen (0) atoms. These atoms then combine with molecular oxygen to form ozone.
Alternatively accept the following equations with the correct molecular formulae. No mark to be assigned if molecular formulae are not correct, when only the equation is written.
Detailed Answer:
Ozone is a triatomic gas formed of three atoms of oxygen (03). Ozone layer absorbs the ultraviolet radiations of the sun and prevent them from reaching the earth.
(B) Why is it essential for all living beings? State the cause for the depletion of this gas. (3)
Answer:
Ozone shields the surface of the earth / protects living organisms from ultraviolet (UV) radiation released by the sun.
Chlorofluorocarbons (CFCs) which are used as refrigerants / in fire extinguishers lead to depletion of ozone layer.
Related Theory
Ozone is present throughout the stratosphere. This rich zone of ozone in the stratosphere is called ozone layer or ozonosphere. Ozone hole was first discovered over Antartica in 1985. The thinning of ozone layer allows more UV radiations to pass through it which then strike the earth. These cause harmful effects an human beings, animals and plants.
In 1987, the UNEP succeeded in forgoing an agreement between nations to limit CFCs production to half the level. It was also recommond that alternate technology will be developed to replace the use of CFCs.
SECTION – C
This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (A. B and C)
Parts A and B are compulsory. However, an internal choice has been provided in part C
Question 14.
Sahil performed an experiment to study the inheritance pattern of genes. He crossed tall pea plants (TT) with short pea plants (tt) arid obtained all tall plants in F1 generation.
(A) What will be set of genes present in the F1 generation?
(B) Give reason why only tall. plants are observed in F1 progeny.
(C) When F1 plants were self – pollinated, a total of 800 plants were produced. How many of these would be tall, medium height or short plants? Give the genotype of F2 generation.
OR
When F1 plants were cross – pollinated with plants having tt genes, a total of 800 plants were produced. How many of these would be tall, medium height or short plants? Give the genotype of F2 generation.
Answer:
(A) Tt
(B) Traits Like ‘T’ are called dominant traits, while those that behave like ‘t’ are called recessive traits./Alternativel accept the definition of dominant and recessive traits with examples of T and t respectively /Alternatively accept the Law of Dominance with examples of T and t.
(C) Out of 800 plants 600 plants will be tall and 200 plants will be small 1 TT: 2Tt: 1 tt
OR
In the cross between Tt × t, 400 Tall. (Tt) and 400 short (tt) plants will be produced. 1 Tt: 1 tt
Concept Applied
Cross between the pea plants with one pair of contrasting characters is called a monohybrid cross. Here the contrasting character are the tallness and shortness of pea plants.
The set of genes in the F1 generation is Tt The F1 generation possess one factor of inheritance from each parent plant which was carried in gametes and ¡s dominant trait. Since all the plants in the F1 generation have the factors Tt. so all of them are toll. When F1 progeny was allowed to be self-pollinated. both the parental traits were expressed in definite proportion in F2 generation.
OR
Monohybrid cross between F1 plants (Tt) with plants having tt genes.
There will be 400 tall (Tt) and 400 short (tt) plants and there wilt be no medium height plants.
Question 15.
Ansari Sir was demonstrating an experiment in his class with the setup as shown in the figure below.
A magnet is attached to a spring. The magnet can go in and out of the stationary coil.
He lifted the Magnet and released it to make it oscillate through the coil. Based on your understanding of the phenomenon, answer the following questions.
(A) What is the principle which Ansari Sir is trying to demonstrate?
(B) What will be observed when the Magnet starts oscillating through the coil. Explain the reason behind this observation.
(C) Consider the situation where the Magnet goes in and out of the coil. State two changes which could be made to increase the deflection in the galvanometer.
OR
Is there any difference in the observations in the galvanometer when the Magnet swings in and then out of the stationary coil? Justify your answer.
Answer:
(A) Sir is trying to demonstrate the principle of Electromagnetic induction.
(B) There will be induced current in the coil due to relative motion between the magnet and the coil. Changing the magnetic field around the coil generates induced current.
(C) Using a stronger magnet, using a coil with more number of turns.
OR
- When the magnet moves into the coil, the galvanometer shows a momentary deflection towards one side say left.
- When the magnet moves out of the coil, the ammeter shows a momentary deflection now towards right,
- This is due to changing magnetic field /flux associated with the coil as the magnet moves in and out.
- Alternatively, the flux increases when the magnet goes in and it decreases when the magnet goes out.
Detailed Answer: (A)
The process by which a changing magnetic field in a conductor induces a current in another conductor is calLed electromagnetic induction. In practice, we can induce the current in a coil either by moving it in a magnetic field or by changing the magnetic field around it.
Related Theory
Motion of a magnet with respect to a coil produces an induced potential difference across its ends and as a result, an induced current is set in the circuit, when the magnet is brought nearer to the coil, a greater number of magnetic field lines, pass through the coil so the magnetic field around the coil increases. The magnetic field of the coil decreases if the magnet is moved away from the coil.
The magnitude of induced current depends on the strength of the magnet, the number of turns in the coil and the speed of the movement of magnet The induced current is found to be highest when the direction of motion of the coil is at right angle to the magnetic field.
OR
When the north pole of the magnet is brought towards the coil, a current flours and the galvanometer shows deflection to wards the right (a). If the magnet is moved away from the coil, the current flows in a direction opposite and the galvanometer shows deflection towards left (fig b).
